Abstract
Background: Bromodomain and extra-terminal domain (BET) family proteins represent a novel target class for patients (pts) with myeloid malignancies. Pharmacologic inhibition of BET proteins transcriptionally downregulates critical pro-survival and anti-apoptotic genes. We hypothesized that combination of BET inhibitor (BETi) with hypomethylating agent (HMA) azacitidine (AZA) could lead to clinical benefit for high-risk (HR) pts with R/R MDS and AML.
Methods: We conducted an investigator-initiated, single-center, phase I, 3+3 dose-escalation and cohort expansion study of PLX51107 (BETi) + AZA in pts with R/R HR MDS (intermediate-2 score or >10% blasts) or R/R AML. PLX51107 was administered PO on days 1-21 and AZA 75 mg/m2 IV on days 8-14 of a 28-day cycle. Dose-escalation phase of PLX51107 doses included: 40mg (n=4), 80mg (n=3), and 120mg; ultimately, no formal MTD was reached, therefore the 120mg dose was administered to the remaining 30 pts treated on study.
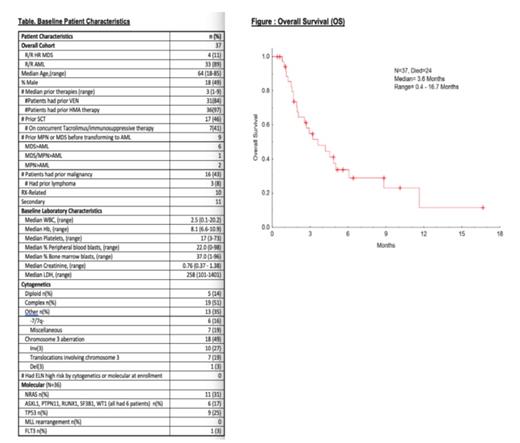
Results: 37 pts were treated [R/R AML (n=33); R/R HR MDS (n= 4)]. Baseline characteristics included in Table. Median age was 64 years [18-85 years]. 51% female. Baseline cytogenetics: Notably, 18 pts (49%) had chromosome (chr) 3 abnormalities (abnl) [(either alone or with complex cytogenetics) with 15/18, or 89%, + for EVI1/MECOM gene rearrangement by FISH. 81-gene panel Next Generation Sequencing (NGS) showed: NRAS (n=11); TP53 (n=9); ASXL1, PTPN11, RUNX1, SF3B1, & WT1 (n=6 each). Median prior # therapies = 3 [1-9]; 97% had prior HMA therapy; 84% had prior venetoclax (VEN).17 (46%) pts had prior stem cell transplant (SCT); 7 of whom were on concurrent active immunosuppressive therapy for GVHD prophylaxis at time of enrollment and continued on study (n=6 tacrolimus; n=1 sirolimus). 9 had prior myeloproliferative neoplasm (MPN) or MDS preceding AML. 16 (43%) had prior malignancy (including lymphoma n=3). Toxicities: Median # cycles on therapy = 2 [1-19+]. Most common grade 1/2 non-hematologic toxicities: fatigue 3%; cholecystitis 3% and vomiting 3%. Notable grade 3/4 non-hematologic toxicities: 9 (24%) pts had elevated bilirubin/liver function tests (n=2 Grade 4 and n=7 Grade 3) and n=24 had infections. Hematologic toxicities were n=7 (4 Grade 3, 3 Grade 4) anemia; n=6 (all Grade 4) thrombocytopenia, n=3 (2 Grade 3, 1 Grade 4) neutropenia.
Outcomes: Overall Response Rate (ORR) = 8/37 (22%): complete remission with incomplete platelet counts (CRp) (n=1); morphological leukemia-free state (MLFS) (n=2) (both MLFS responders had chr 3 abnl); hematologic improvement (HI) (n=5). Additionally, 5 other pts had >50% bone marrow blast reduction. 4 pts were able to stay on study ≥6 months, with one patient staying on active therapy with ongoing clinical benefit for ≥1 year (16.7 months+). Notably, 10/13 (77%) of all of these responding pts occurred in prior VEN-treated pts. 6/13 (46%) of all responses occurred in RUNX1 or NRAS/KRAS family-mutated pts. Median time on study was 4.8 months [0.4-16.7]. Median overall survival was 3.6 [0.4 - 16.7] months (Figure). One remarkable responder, a 70 year-old woman with R/R AML and extensive leukemia cutis, (prior VEN-based therapy), achieved CRp and almost complete resolution of all skin lesions with no major toxicities after 3 cycles of therapy.
Laboratory correlative analysis: RNA-Seq analysis of mononuclear cells harvested on- treatment (day 3) vs pre-treatment demonstrated markedly greater fold changes in mRNA expressions in the complete responders, with downregulation of MYC, BCL2, IL7R and CDK6 genes and upregulation of HEXIM, CD93, DCXR and CDKN1A genes. Immunoblot analyses confirmed reduction in the protein levels of c-Myc, CDK6, BCL2 and BCL-xL and induction of BRD4 and HEXIM1 protein levels in the responding pts.
Conclusions: In a heavily pre-treated, high-risk group of pts with R/R HR MDS and AML, with 49% chr 3 abnl, 46% prior SCT, and 25% TP53-mutated, we demonstrate that the combination of BETi and HMA is safe, well-tolerated, and results in modest clinical benefit predominantly in prior VEN-treated pts. Future directions may include investigation of novel BETi combinations in VEN-naïve pts, further investigation into high-risk subsets of pts with chr 3 abnl/EVI1/MECOM rearrangements, RUNX1, and RAS-family mutated pts, and further clinical/translational investigation of BETi activity in leukemia cutis/skin lesions in myeloid malignancies. ClinicalTrials.gov Identifier: NCT04022785.
Pemmaraju: Sager Strong Foundation: Other; Samus: Other, Research Funding; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Plexxicon: Other, Research Funding; Daiichi Sankyo, Inc.: Other, Research Funding; Cellectis S.A. ADR: Other, Research Funding; CareDx, Inc.: Consultancy; Aptitude Health: Consultancy; Springer Science + Business Media: Other; DAVA Oncology: Consultancy; Roche Diagnostics: Consultancy; MustangBio: Consultancy, Other; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Celgene Corporation: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; LFB Biotechnologies: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Incyte: Consultancy; Affymetrix: Consultancy, Research Funding; Protagonist Therapeutics, Inc.: Consultancy; Clearview Healthcare Partners: Consultancy; Blueprint Medicines: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Daver: Abbvie: Consultancy, Research Funding; Novartis: Consultancy; Hanmi: Research Funding; Genentech: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Trovagene: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Amgen: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Glycomimetics: Research Funding; Novimmune: Research Funding; Pfizer: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Ravandi: Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; Xencor: Honoraria, Research Funding; Novartis: Honoraria; AstraZeneca: Honoraria; AbbVie: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Prelude: Research Funding. Kadia: AstraZeneca: Other; Genfleet: Other; Astellas: Other; Cellonkos: Other; Ascentage: Other; Sanofi-Aventis: Consultancy; Pulmotech: Other; Pfizer: Consultancy, Other; Novartis: Consultancy; Liberum: Consultancy; Jazz: Consultancy; Genentech: Consultancy, Other: Grant/research support; Dalichi Sankyo: Consultancy; Cure: Speakers Bureau; BMS: Other: Grant/research support; Amgen: Other: Grant/research support; Aglos: Consultancy; AbbVie: Consultancy, Other: Grant/research support. DiNardo: GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Forma: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Agios/Servier: Consultancy, Honoraria, Research Funding; Novartis: Honoraria; Takeda: Honoraria; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding. Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Burger: Beigene: Research Funding, Speakers Bureau; TG Therapeutics: Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Novartis: Other: Travel/Accommodations/Expenses, Speakers Bureau; Pharmacyclics LLC: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Gilead: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; AstraZeneca: Consultancy; Janssen: Consultancy, Other: Travel/Accommodations/Expenses, Speakers Bureau. Short: Novartis: Honoraria; NGMBio: Consultancy; Jazz Pharmaceuticals: Consultancy; AstraZeneca: Consultancy; Astellas: Research Funding; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria. Alvarado: Astex Pharmaceuticals: Research Funding; BerGenBio: Research Funding; CytomX Therapeutics: Consultancy; Daiichi-Sankyo: Research Funding; FibroGen: Research Funding; Jazz Pharmaceuticals: Research Funding; MEI Pharma: Research Funding; Sun Pharma: Consultancy, Research Funding. Jain: TG Therapeutics: Honoraria; Beigene: Honoraria; Precision Biosciences: Honoraria, Research Funding; Incyte: Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Research Funding; Pfizer: Research Funding; AstraZeneca: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Pharmacyclics: Research Funding; Janssen: Honoraria; Fate Therapeutics: Research Funding; Aprea Therapeutics: Research Funding; Servier: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding. Verstovsek: Promedior: Research Funding; PharmaEssentia: Research Funding; NS Pharma: Research Funding; Ital Pharma: Research Funding; Incyte Corporation: Consultancy, Research Funding; Gilead: Research Funding; Genentech: Research Funding; CTI BioPharma: Research Funding; Celgene: Consultancy, Research Funding; Blueprint Medicines Corp: Research Funding; AstraZeneca: Research Funding; Novartis: Consultancy, Research Funding; Protagonist Therapeutics: Research Funding; Roche: Research Funding; Sierra Oncology: Consultancy, Research Funding; Constellation: Consultancy; Pragmatist: Consultancy. Issa: Novartis: Consultancy, Research Funding; Kura Oncology: Consultancy, Research Funding; Syndax Pharmaceuticals: Research Funding. Khoury: Kiromic: Research Funding; Angle: Research Funding; Stemline Therapeutics: Research Funding. Konopleva: Ablynx: Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Forty Seven: Other: grant support, Research Funding; Cellectis: Other: grant support; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Rafael Pharmaceuticals: Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Stemline Therapeutics: Research Funding; Sanofi: Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; KisoJi: Research Funding. Kantarjian: Astra Zeneca: Honoraria; Aptitude Health: Honoraria; Jazz: Research Funding; Daiichi-Sankyo: Research Funding; Astellas Health: Honoraria; Ipsen Pharmaceuticals: Honoraria; AbbVie: Honoraria, Research Funding; Ascentage: Research Funding; Amgen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Immunogen: Research Funding; BMS: Research Funding; KAHR Medical Ltd: Honoraria; NOVA Research: Honoraria; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Borthakur: Takeda: Membership on an entity's Board of Directors or advisory committees; ArgenX: Membership on an entity's Board of Directors or advisory committees; Ryvu: Research Funding; Astex: Research Funding; University of Texas MD Anderson Cancer Center: Current Employment; Protagonist: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy.